- MAIA Intends to initiate its second Phase 2 go-to-market trial – THIO-102 - evaluating THIO with atezolizumab, pembrolizubmab and other checkpoint inhibitors in multiple tumor types, including small cell lung cancer, liver cancer and colorectal cancer
MAIA Biotechnology, Inc. (NYSE American: MAIA) announced today it intends to initiate its second Phase 2 go-to-market trial evaluating THIO, the world’s first telomere-targeting agent, in patients with four cancer indications. The FDA has awarded MAIA’s lead anti-cancer agent THIO two Orphan Drug Designations, based on the preclinical efficacy data, for liver (hepatocellular carcinoma) and small cell lung cancer models. The trial is designed to evaluate THIO in sequential combination with the immunotherapies pembrolizumab or atezolizumab, which are the most used checkpoint inhibitors in Oncology. A third immunotherapy checkpoint inhibitor could be added later such as nivolumab, durvalumab, dostarlimab, etc.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230213005224/en/
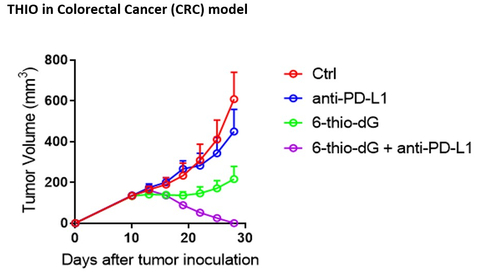
Figure Legend: THIO (6-thio-dG) is highly synergistic and curative with anti-PD-L1 agent atezolizumab in MC 38 cell-based syngeneic model of Colorectal Cancer (CRC). Treatment with THIO sequentially followed by atezolizumab results in highly potent anticancer effect, as compared to the effects of atezolizumab alone. THIO administration converts immunologically “cold non-responsive” CRC tumor into “hot and responsive to anti-PD-L1 agent atezolizumab”. (Photo: Business Wire)
The trial previously demonstrated positive and encouraging preclinical results in colorectal, liver, and small cell lung cancer models.
Treatment with THIO sequentially followed by pembrolizumab results in highly potent anticancer effect, as compared to the effects of pembrolizumab alone.
THIO converts immunologically “cold” non-responsive SCLC tumor into “hot” and responsive to pembrolizumab.
Treatment with THIO in combination with IR and atezolizumab results in a complete regression of aggressive HCC tumors. At the same time, the combination of IR and atezolizumab is just partially efficacious.
“The THIO-102 trial is operationally on track to begin enrolling patients later this year,” said Mihail Obrocea, MD, MAIA’s Chief Medical Officer. “Based on the data generated on these indications, we are targeting accelerated approvals in these tumor types; in addition, we have now added a fourth arm which includes solid tumors of all types, that will serve as a signal generation arm; we will include telomerase positive breast, prostate, gastric, pancreatic, ovarian, along with potentially other tumor types.”
“These are patients that are facing very limited treatment options, usually chemotherapy with minimal efficacy and high toxicity,” said MAIA Chairman and Chief Executive Officer Vlad Vitoc, M.D. “THIO sequenced with an immune checkpoint inhibitor has demonstrated complete tumor regression in several cancer preclinical models. We are very confident THIO can substantially improve on the limited clinical efficacy shown so far by atezolizumab, pembrolizumab and others. This go-to-market trial - THIO 102 - may provide THIO with more than 9 additional indications. Our existing trial with non-small cell lung cancer is another indication, potentially giving THIO more than 10 indications in total. Most oncology compounds at this stage of development have only one. We have 10 shots on goal!”“
About THIO-102
THIO-102 is an upcoming multicenter, open-label, go-to-market Phase 2 trial designed to evaluate the safety and efficacy of THIO administered in sequence with anti-PD-1 or anti-PD-L1 in patients with telomerase (+) tumors. Following an innovative basket/umbrella design, the trial is comprised of four baskets: small cell lung cancer, liver, colorectal, and solid tumors all types. The first basket is in first line treatment of small cell lung cancer, where MAIA plans to evaluate THIO added to the current standard of care, EP + atezolizumab. In the remaining arms, MAIA plans to evaluate THIO in sequential combination with atezolizumab or pembrolizumab; the objective is to select the best combination by tumor type in Part A and expand into Part B that will include multiple Phase 2 pivotal arms seeking accelerated approvals.
About THIO
THIO is a telomere-targeting agent currently in clinical development to evaluate its activity in non-small cell lung cancer (NSCLC). Telomeres play a fundamental role in the survival of cancer cells and their resistance to current therapies. THIO is being developed as a second or higher line of treatment for NSCLC for patients that have progressed beyond the standard-of-care regimen of existing checkpoint inhibitors.
About MAIA Biotechnology, Inc.
MAIA is a clinical-stage biopharmaceutical company developing targeted immunotherapies for cancer. The Company’s lead program is THIO, a potential first-in-class cancer telomere targeting agent in clinical development for the treatment of patients with telomerase-positive cancers. For more information, please visit www.maiabiotech.com.
Forward Looking Statements
This press release includes forward-looking statements including, but not limited to, statements related to the closing of the offering and the expected use of proceeds, development of drug candidates, our operations and business strategy, our expected financial results, and corporate updates. The forward-looking statements contained in this press release are based on management’s current expectations and are subject to substantial risks, uncertainty and changes in circumstances. Actual results may differ materially from those expressed by these expectations due to risks and uncertainties, including, among others, those related to our ability to obtain additional capital on favorable terms to us, or at all, including, without limitation, to fund our current and future preclinical studies and clinical trials and the success, timing and cost of our drug development program and our ongoing or future preclinical studies and clinical trials, including, without limitation, the possibility of unfavorable new clinical and preclinical data and additional analyses of existing data, that the risks that prior clinical and preclinical results may not be replicated, and risks associated with the current coronavirus pandemic. Forward-looking statements speak only as of the date of this press release, and we undertake no obligation to review or update any forward-looking statement except as may be required by applicable law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230213005224/en/
Contacts
Investor Inquiries
MAIA Biotechnology
Joe McGuire
Chief Financial Officer
jmcguire@maiabiotech.com
904-228-2603
ICR Westwicke
Stephanie Carrington
Stephanie.carrington@westwicke.com
646-277-1282













