Support in Place via Collaboration with Biotech Giants Regeneron and Bristol Myers Squibb for Checkmate Pharmaceuticals (NASDAQ: CMPI)
Checkmate Pharmaceuticals, Inc. (OTCMKTS:CMPI)
We announced our intention to broaden our vidutolimod clinical development program into non-melanoma skin cancers in combination with Libtayo® , under a collaboration agreement with Regeneron”
CAMBRIDGE, MASSACHUSETTS, UNITED STATES, June 8, 2021 /EINPresswire.com/ -- Support in Place via Collaboration with Biotech Giants Regeneron and Bristol Myers Squibb for Checkmate Pharmaceuticals (NASDAQ: CMPI)— Barry Labinger, President
Proprietary Technology Using the Immune System to Fight Cancer.
Clinical Trials Underway on Several Different Cancer Types.
Collaborative Agreements with Regeneron and Bristol Myers Squibb.
Plans to Broaden the Scope of Applications to More Cancer Types.
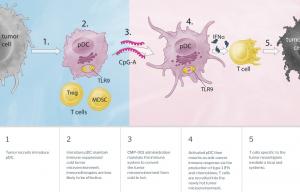
Checkmate Pharmaceuticals (NASDAQ: CMPI) is a clinical stage biotechnology company focused on developing its proprietary technology to harness the power of the immune system to combat cancer. CMPI product candidate, Vidutolimod (CMP-001), is an advanced generation Toll-like receptor 9 (TLR9) agonist, delivered as a biologic virus-like particle utilizing a CpG-A oligodeoxynucleotide as a key component, designed to trigger the body’s innate immune system to attack tumors in combination with other therapies.
CMPI is a NASDAQ listed company with an attractive share structure of only 21,625,891 shares outstanding.
CMPI Presents at the Jefferies 2021 Virtual Healthcare Conference
On May 20thCMPI announced that Barry Labinger, CEO, would present at the Jefferies Virtual Healthcare Conference from 2:00-2:25pm ET on Tuesday, June 1, 2021. CMPI also announced it would host 1x1 investor meetings during the conference.
A live webcast of the presentation can be accessed under “Events & Presentations” in the Investors section of the CMPI company website at www.checkmatepharma.com. An archived copy of the webcast will be available on the website for approximately 30 days after the event.
CMPI First Quarter 2021 Financial Results & Update on Recent Progress
On May 13thCMPI announced first quarter 2021 financial results and provided an update on recent progress.
Since the start of 2021, CMPI has initiated patient dosing in its core clinical trials evaluating Vidutolimod (CMP-001) in combination with PD-1 blockade in melanoma and head and neck cancer. CMPI also recently announced its intention to broaden its Vidutolimod clinical development program into non-melanoma skin cancers in combination with Libtayo® (cemiplimab), under a collaboration agreement with Regeneron.
Recent Progress
Vidutolimod Clinical Updates
• Year to date, CMPI has initiated patient dosing across all three of its core clinical trials evaluating Vidutolimod. A randomized Phase 2/3 trial of Vidutolimod in combination with nivolumab vs. nivolumab monotherapy in first-line metastatic or unresectable melanoma. A Phase 2 trial of Vidutolimod in combination with nivolumab in anti-PD-1 refractory advanced melanoma. Both melanoma trials are supported by a clinical collaboration with Bristol Myers Squibb.A Phase 2 trial of Vidutolimod in combination with pembrolizumab in recurrent or metastatic squamous cell head and neck cancer. Initial data in a subset of patients are expected before the end of 2021.
• New translational data were presented from CMPI Phase 1b trial of Vidutolimod in combination with pembrolizumab in patients with melanoma refractory to PD-1 blockade at the 2021 American Association for Cancer (AACR) Annual Meeting.
Collaboration and New Indication Expansion
• In May, CMPI announced the planned expansion of the development program for Vidutolimod into non-melanoma skin cancers supported by a clinical supply agreement with Regeneron to evaluate the combination of Vidutolimod and Libtayo® (cemiplimab). The companies will collaborate on a Phase 2, proof of concept, multi-indication trial with patient cohorts in anti-PD-1 naïve and anti-PD-1 refractory cutaneous squamous cell carcinoma and anti-PD-1 refractory Merkel cell carcinoma.
First Quarter 2021 Financial Results
• Research and development expenses (R&D): R&D expenses for the first quarter of 2021 were $10.4 million, compared to $6.3 million for the same period in the prior year. This increase reflected a milestone payment of $2.0 million in the first quarter of 2021 triggered by initiation of patient dosing in CMPI Phase 2, first-line melanoma trial, as well as increases in personnel and operating expense for the planning and initiation of additional clinical trials with Vidutolimod.
• General and administration expenses (G&A): G&A expenses for the first quarter of 2021 were $3.8 million, compared to $1.5 million for the same period in the prior year. This increase was primarily attributable to increases in personnel and operating expense incurred in connection with CMPI operating as a publicly traded company.
• Cash, cash equivalents and investments: Cash, cash equivalents and available-for-sale investments were $111.5 million as of March 31, 2021.
For more information on Checkmate Pharmaceuticals (CMPI) visit: www.checkmatepharma.com.
DISCLAIMER: FrontPageStocks/CorporateAds.com (CA) is a third-party publisher and news dissemination service provider. FPS/CA is NOT affiliated in any manner with any company mentioned herein. FPS/CA is news dissemination solutions provider and are NOT a registered broker/dealer/analyst/adviser, holds no investment licenses and may NOT sell, offer to sell or offer to buy any security. FPS/CA’s market updates, news alerts and corporate profiles are NOT a solicitation or recommendation to buy, sell or hold securities. The material in this release is intended to be strictly informational and is NEVER to be construed or interpreted as research material. All readers are strongly urged to perform research and due diligence on their own and consult a licensed financial professional before considering any level of investing in stocks. All material included herein is republished content and details which were previously disseminated by the companies mentioned in this release or opinion of the writer. FPS/ CA is not liable for any investment decisions by its readers or subscribers. Investors are cautioned that they may lose all or a portion of their investment when investing in stocks. FPS/CA has been compensated $500 by a third for dissemination of this Article.
Disclaimer/Safe Harbor:
These news releases and postings may contain forward-looking statements within the meaning of the Securities Litigation Reform Act. The statements reflect the Company’s current views with respect to future events that involve risks and uncertainties. Among others, these risks include the expectation that any of the companies mentioned herein will achieve significant sales, the failure to meet schedule or performance requirements of the companies’ contracts, the companies’ liquidity position, the companies’ ability to obtain new contracts, the emergence of competitors with greater financial resources and the impact of competitive pricing. In the light of these uncertainties, the forward-looking events referred to in this release might not occur.
SOURCE: CorporateAds.com
CMPI
Checkmate Pharmaceuticals, Inc.
+1 617-682-3625
email us here
Visit us on social media:
Twitter
LinkedIn
$CMPI IPO