Analysis of sera from Phase 1/2 booster study demonstrates 54- and 47-fold increases in geometric mean neutralizing antibody titers against the Omicron variant at Day 29 for ARCT-154 (5 mcg) and ARCT-165 (5 mcg) respectively
Arcturus Therapeutics Holdings Inc. (the “Company”, “Arcturus”, Nasdaq: ARCT), a leading clinical-stage messenger RNA medicines company focused on the development of infectious disease vaccines and significant opportunities within liver and respiratory rare diseases, today announced new data from clinical development programs for ARCT-154 and ARCT-165, its investigational, next-generation, self-amplifying mRNA vaccine candidates targeting variants of concern.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220125005602/en/
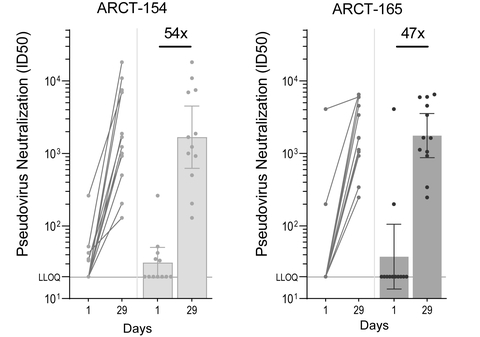
Figure 1: Pseudovirus (Omicron variant, research use) MNT assay results. Antibody titers corresponding to 50% viral inhibition (ID50) in trial participants at Day 1 (prior to boosting) and Day 29 after boosting with ARCT-154 (left; n = 12/12) and ARCT-165 (right; n = 12/12). The bar graphs show the geometric means of neutralization titers, with 95% confidence intervals. The multiples are fold rises of neutralizing antibody titers on Day 29 over Day 1 values. ID¬50: half-maximal inhibitory dose; LLOQ: lower limit of quantitation. (Graphic: Business Wire)
New data from the Phase 1/2 booster trial, sponsored by Arcturus and currently ongoing in U.S. and Singapore, demonstrated that ARCT-154 and ARCT-165, when administered in low doses (5 mcg) at least five months following initial vaccination with Comirnaty®, yielded robust increases of 54- and 47-fold, respectively, in neutralizing antibody responses against the Omicron variant. These findings provide additional data to recently updated results from the Phase 1/2 demonstrating robust neutralizing antibody responses against SARS-CoV-2 D614G strain as well as several variants of concern, and is expected to support the expansion of clinical development of the candidates to evaluate their potential as booster vaccines.
“Arcturus’ lower dose STARR™ self-amplifying mRNA vaccine technology continues to generate highly encouraging clinical booster data, increasing the platform’s potential to address endemic markets for COVID and other infectious diseases,” said Joseph Payne, President and CEO of Arcturus Therapeutics. “Demonstration of high levels of neutralizing antibody responses against a broad range of variants, including Omicron, suggests that our self-amplifying mRNA vaccine platform has the potential to combat not only the present variants of concern, but also emerging variants of SARS-CoV-2.”
Twenty four (24) participants, divided equally in the ARCT-154 and ARCT-165 booster groups, received 5 micrograms of ARCT-154 or ARCT-165 following primary vaccination with Comirnaty® at least 5 months earlier. All participants in the booster trial were below 65 years of age at the time of receiving the booster dose. Figure 1 shows the neutralizing antibody geometric mean titers against the Omicron strain for the ARCT-154 and the ARCT-165 groups on Day 29 post-boost compared to pre-boost titer, as measured by an exploratory microneutralization titer (MNT) assay (Moore Laboratory, National Institute for Communicable Diseases and University of the Witwatersrand, South Africa).
About Arcturus Therapeutics
Founded in 2013 and based in San Diego, California, Arcturus Therapeutics Holdings Inc. (Nasdaq: ARCT) is a clinical-stage mRNA medicines and vaccines company with enabling technologies: (i) LUNAR® lipid-mediated delivery, (ii) STARR™ mRNA Technology and (iii) mRNA drug substance along with drug product manufacturing expertise. Arcturus’ diverse pipeline of RNA therapeutic and vaccine candidates includes mRNA vaccine programs for SARS-CoV-2 (COVID-19) and influenza, and other programs to potentially treat ornithine transcarbamylase (OTC) deficiency, and cystic fibrosis along with partnered programs including glycogen storage disease type III (GSD III), hepatitis B virus (HBV), and non-alcoholic steatohepatitis (NASH). Arcturus’ versatile RNA therapeutics platforms can be applied toward multiple types of nucleic acid medicines including messenger RNA, small interfering RNA, replicon RNA, antisense RNA, microRNA, DNA, and gene editing therapeutics. Arcturus’ technologies are covered by its extensive patent portfolio (with patents and patent applications issued in the U.S., Europe, Japan, China and other countries). Arcturus’ commitment to the development of novel RNA therapeutics has led to collaborations with Janssen Pharmaceuticals, Inc., part of the Janssen Pharmaceutical Companies of Johnson & Johnson, Ultragenyx Pharmaceutical, Inc., Takeda Pharmaceutical Company Limited, CureVac AG, Duke-NUS Medical School, and the Cystic Fibrosis Foundation. For more information visit www.ArcturusRx.com. In addition, please connect with us on Twitter and LinkedIn.
Forward Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact included in this press release, are forward-looking statements, including those regarding strategy, future operations, the expectations for or likelihood of success of any collaborations, the likelihood of success (including safety and efficacy) of the Company’s platform or pipeline (including ARCT-154 and ARCT-165), the Company’s efforts to develop a vaccine against COVID-19 and therapeutic potential thereof based on the Company’s mRNA therapeutics, the planned initiation, design or completion of clinical trials, the likelihood that the Company will obtain clearance from regulatory authorities to proceed with future planned clinical trials, the likelihood that preclinical or clinical data will be predictive of future clinical results (including with respect to safety, immunogenicity and efficacy), the likelihood that a preliminary, interim or partial data set will be representative of a complete or larger data set, the likelihood that clinical data will be sufficient to support further clinical development, for regulatory approval or will be completed in time to submit an application for regulatory approval within a particular timeframe, the likelihood that a patent will issue from any patent application and the impact of general business and economic conditions. Arcturus may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in any forward-looking statements such as the foregoing and you should not place undue reliance on such forward-looking statements. These statements are only current predictions or expectations, and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements, including those discussed under the heading "Risk Factors" in Arcturus’ most recent Annual Report on Form 10-K, and in subsequent filings with, or submissions to, the SEC, which are available on the SEC’s website at www.sec.gov. Except as otherwise required by law, Arcturus disclaims any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events or circumstances or otherwise.
Trademark Acknowledgements
The Arcturus logo and other trademarks of Arcturus appearing in this announcement, including LUNAR® and STARR™, are the property of Arcturus. All other trademarks, services marks, and trade names in this announcement are the property of their respective owners.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220125005602/en/
Contacts
IR and Media Contacts
Arcturus Therapeutics
Deepankar Roy, Ph.D.
(858) 900-2682
IR@ArcturusRx.com
Kendall Investor Relations
Carlo Tanzi, Ph.D.
(617) 914-0008
ctanzi@kendallir.com













